We would like to make the public aware of a confirmed case of Leptospirosis in a dog which we believe to be related to the recent flooding in the local area. The case has been confirmed by laboratory testing as L. Copenhageni, a strain of Leptosporosis that is COVERED BY BOTH THE L2 AND L4 VACCINATION.
This is the second confirmed case of Leptospirosis in the last six weeks, with two suspected cases that haven’t been tested at the lab.
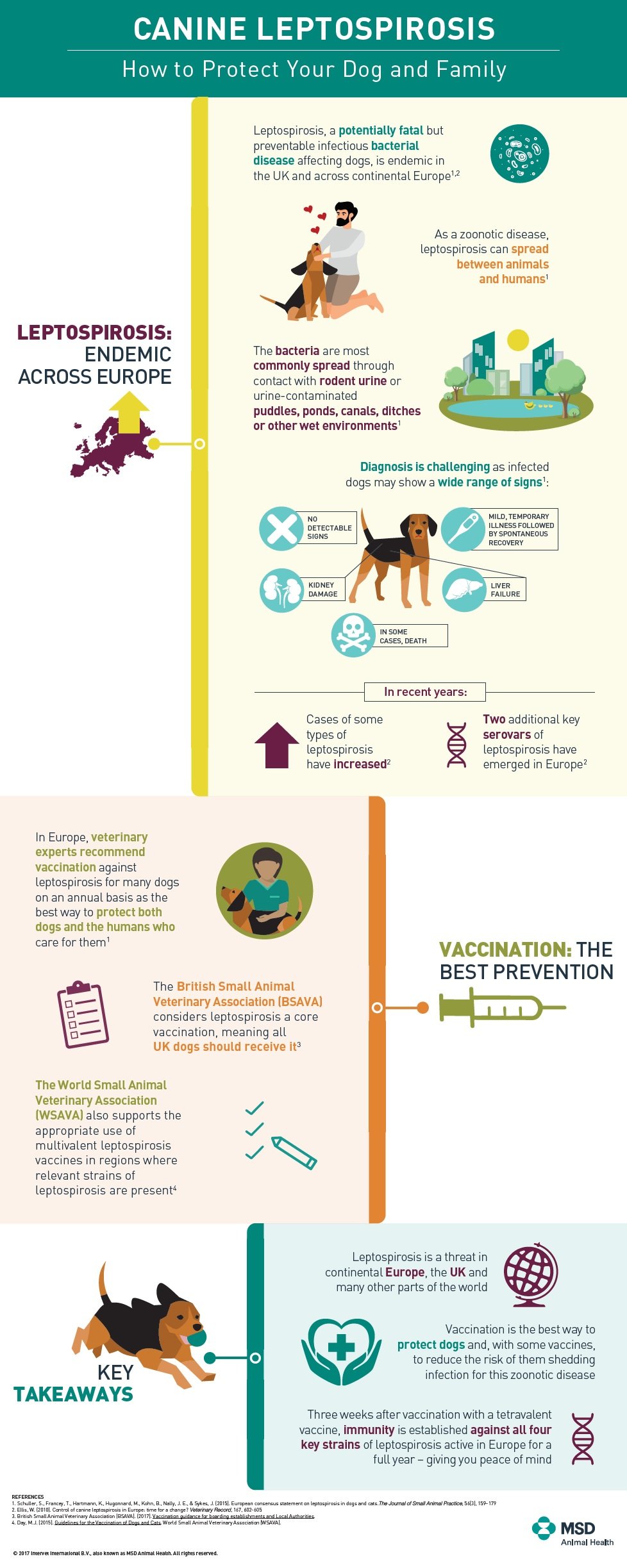
Leptospirosis is a spirochaete bacteria which can survive for months in water and moist soil. Rats are the usual host of the bacteria but other mammals (eg. dogs /cats) and people can become accidentally infected. Signs vary from mild to severe and potentially fatal. Multiple organs within the body may be involved (particularly the liver and kidneys) and the affected animal can be infectious to other animals and humans via their urine.
Leptospirosis is considered a seasonal disease, with human and animal outbreaks linked to heavy rainfall or flooding.
Animals can be successfully treated if the disease is caught in the early stages but sadly some cases are fatal.
There is a vaccine that can help to prevent this disease in dogs and we encourage dog owners to check that their animals are up to date with the new generation vaccination (L4) as the older generation (L2) vaccines will not protect as many strains of Leptospirosis that are currently being found across the UK.
Vaccination involves a primary course of 2 injections 4 weeks apart followed by an annual vaccination. The annual booster vaccination is important for the Leptosporosis cover as the immunity does wane much faster than the other core vaccinations.
LEPTOSPIROSIS THE DISEASE
Leptospirosis is a potentially fatal, but preventable infectious bacterial infection. It can impact most mammals but is particularly devastating for dogs and humans. It is endemic in the UK and becoming more prevalent across Europe.
It is most commonly spread through water or soil contained by the urine of infected wildlife (predominantly rats and other rodents). These high risk areas include lakes, streams and ditches. Diagnosis is challenging as infected dogs may show a variety of signs – some may not show any signs, while some have a mild, temporary illness then spontaneously recover and others experience kidney damage, liver failure and even death.
In recent years two emerging strains of the disease have become more widespread across the UK. In response to growing concern among experts that existing vaccines no longer provided adequate protection, MSD Animal Health introduced Nobivac L4, the first Leptospirosis vaccine providing broad protection against all four strains active in the UK and Europe. Since its launch in 2013, Nobivac L4 has been widely adopted in the UK and has become one of the most commonly used dog vaccines with several million doses administered. Zoetis have subsequently released a version called Versican L4.
IMPORTANCE OF VACCINATION
Veterinary experts recommend the majority of animals in endemic areas are protected against canine Leptospirosis through vaccination. Given its increasing prevalence, Leptospirosis vaccination is recommended for UK dogs on an annual basis. The British Small Animal Veterinary Association (BSAVA) defines Leptospirosis as a core vaccination – a vaccine that protects animals from severe, life-threatening diseases that have global distribution and which ALL dogs and/or cats, regardless of circumstances or geographical location, should receive. Further, with the increase in animals travelling across regions, there is a growing need to vaccinate outside of endemic areas as well.
The British Small Animal Veterinary Association defines Leptospirosis as a core vaccination.
DEMONSTRATED SAFETY AND TOLERABILITY
As a practice we are confident in the safety of Nobivac L4. Similar to the process for human medicines, the safety of Nobivac L4 was established through comprehensive clinical research studies. Multiple regulatory agencies have reviewed the Nobivac L4 clinical research data and deemed the vaccine safe for sale in their countries when used according to the product label. Global post-marketing surveillance further reinforces the safety of Nobivac L4. In four years of post-marketing surveillance, the overall reporting rate for suspected adverse events is classified as ‘rare’ (more than 1 but less than 10 per 10,000 doses), and the incidence for serious adverse events is categorized as ‘very rare’ (less than 1 per 10,000 doses).
We understand the importance of knowing the potential adverse reactions that could occur with any medicine or vaccine administered to our pets and strive to be transparent about any possible risks associated with the products we use and supply.
The most common adverse events associated with Nobivac L4 in clinical trials include a mild and temporary increase in body temperature (≤ 1°C) and a small, temporary swelling around the injection site. In addition, some puppies may show less activity or a reduced appetite for a few days following vaccination.



